Gedeptin® is a gene-directed enzyme prodrug therapy (GDEPT) that results in the formation of the oncolytic agent within the tumor itself, resulting in tumor cell death while significantly limiting systemic exposure. Preclinical and early-stage human clinical data suggest the GDEPT approach can destroy otherwise refractory cancer cells in a safe and effective manner.
Gedeptin is based upon a replication-deficient adenoviral vector, a well-recognized vaccine platform used in existing vaccines. Examples include the Johnson & Johnson single-shot Covid-19 vaccine. However, instead of coding for the SARS-CoV-2 spike protein, Gedeptin codes for an enzyme derived from E. coli called purine nucleoside phosphorylase (PNP). Gedeptin is injected directly into the tumor mass three times over a period of two days.
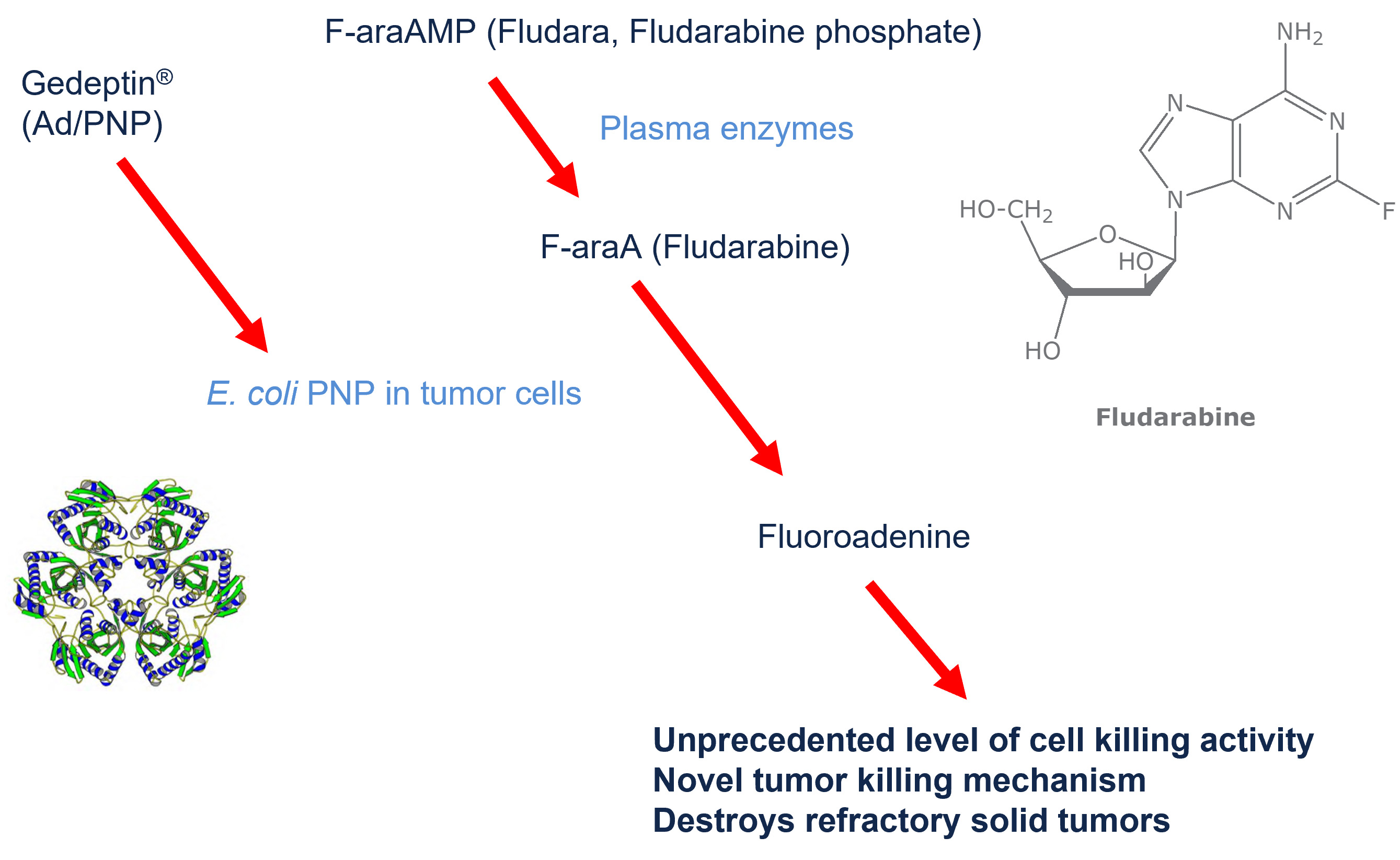
The PNP enzyme, by itself, has no anti-cancer activity, and because it is derived from bacteria, the enzyme is unlikely to have significant metabolic activity in humans. However, the PNP enzyme can be used in combination with purine nucleoside prodrugs in order to generate active chemotherapeutic agents within the tumor cells (in situ).
The purine nucleoside drug, fludarabine phosphate (Fludara®) is approved for the treatment of certain hematological tumors but has shown minimal effectiveness against solid tumors. However, when fludarabine is taken up by cells previously treated with Gedeptin, the absorbed fludarabine is converted, in situ, to the potent cytotoxic agent, fluoroadenine. A cycle of Gedeptin therapy involves the intratumoral administration of three doses of Gedeptin over a two-day period. This is followed by the intravenous administration of fludarabine phosphate, once a day, for the next 3 days. The result, when the Gedeptin-treated cells absorb the fludarabine, is the targeted, site-specific activation of fludarabine to the active “cancer-killing” chemotherapy, fluoroadenine, within the cancer cells themselves.
Fluoroadenine works by blocking DNA synthesis and is particularly effective at killing the rapidly dividing refractory solid tumors dependent on DNA, RNA, and protein synthesis for growth.
About the Gedeptin® Technology Platform
Many common cancers (including prostate, breast, colon, lung, brain, melanoma, pancreas, ovarian, kidney) become untreatable despite the best medical intervention and the highest standard of care and are eventually fatal. Chemotherapeutic agents may be able to destroy these tumors, but many are much too toxic to administer systemically to already debilitated cancer patients. Most conventional anti-cancer drugs in use today derive their anti-tumor specificity from the ability to kill rapidly dividing cells. These drugs are suitable for systemic administration specifically because they are most toxic to cells that are dividing. However, many tumors such as head and neck squamous cell carcinoma (HNSCC) are resistant to treatment because they have a very low growth fraction (i.e., a relatively small percentage of tumor cells dividing at any particular point in time). Compounds toxic to non-proliferating cells generally are not used in the treatment of cancer, because most of the cells in a patient are not proliferating and such compounds have no selectivity when administered systemically.
Among the various gene therapy strategies for cancer treatment, GDEPT (Gene-Directed Enzyme Prodrug Therapy), also known as “suicide gene therapy”, has shown promise. In GDEPT a vector is used to selectively transduce tumor cells with a nonhuman gene, which expresses an enzyme that can convert a non-toxic prodrug into a very toxic antitumor compound. A prodrug is a pharmaceutical compound that remains inactive in its biochemical form until it reaches its target site, such as an organ or tissue, and then undergoes an immediate metabolic breakdown; it thereby releases the molecular compounds of the parent drug, or active ingredients, at the point of delivery to generate optimal results. Because the nonhuman gene is only expressed in tumor tissue, the nontoxic prodrug is only activated in tumor tissue. Therefore, unlike conventional chemotherapy, GDEPT should result in selective killing of tumor cells with little or no systemic toxicity.
GDEPT strategies that produce potent cytotoxic agents (active against nonproliferating and proliferating tumors cells) and that have high bystander activity could have dramatic effects on the treatment of solid tumors. A bystander effect typically refers to the death, altered growth, or damage of cells that have not directly received chemotherapy or irradiation. Earlier GDEPT approaches have had limited efficacy specifically because of poor bystander activity and the inability to destroy non-proliferating tumor cells.
Gedeptin® potentially overcomes previous GDEPT limitations and serves as a robust
platform for development in multiple indications. Gedeptin® consists of a non-replicating adenoviral vector expressing an optimized E. coli purine nucleoside phosphorylase (E. coli PNP) that is injected intratumorally and then followed by intravenous or intratumoral administration of a prodrug.
Among the prodrugs that have been evaluated for use with Gedeptin®, fludarabine phosphate (Fludara®) is of particular interest because (i) it is currently approved by the FDA for use in humans and (ii) it has demonstrated excellent in vivo antitumor activity in murine models when only 2-3% of tumor cells express E. coli PNP. Fludarabine is currently approved by the FDA to treat chronic lymphocytic leukemia but has not been shown to be effective against other solid tumors. But when fludarabine is administered following Gedeptin®, the combination exploits the selective expression of the E. coli PNP gene in tumor cells to utilize fludarabine phosphate as a prodrug, resulting in the localized production of fluoroadenine (F-Ade), a potent cytotoxic compound with pronounced antitumor activity.
In summary, the use of the Gedeptin® plus fludarabine has a number of potential advantages over both conventional and experimental chemotherapeutics. These include:
- Destroys solid tumors resistant to conventional treatments without significant systemic or other toxicity
- Novel tumor-killing mechanism that exhibits synergy with conventional treatment modalities
- Excellent killing of neighboring tumor cells that do not express the enzyme (bystander effect)
- Active against non-proliferating and proliferating tumors cells
- Efficacy at the site of application, thus limiting side effects
- Extensive clinical experience with fludarabine phosphate administered systemically, a licensed chemotherapeutic agent
- High potency of fluoroadenine (F-Ade)
- Safety of the approach.
