Vaccines contain components (antigens) that represent disease-causing microorganisms. Traditional vaccines are often made from weakened or killed forms of the virus or from its surface proteins. Many newer vaccines use recombinant DNA (deoxyribonucleic acid) technology to generate vaccine antigens in bacteria or cultured cells from specific portions of the target pathogen. The generated antigens are then purified and formulated for use in a vaccine. The most successful of these purified antigens have been non-infectious virus-like particles (VLPs) as exemplified by vaccines for hepatitis B (Merck's Recombivax® and GSK's Engerix®) and human papilloma viruses (GSK's Cervarix®, and Merck's Gardasil®). Our approach uses recombinant DNA or recombinant viruses to produce VLPs in the person being vaccinated. In previous human clinical trials of our HIV vaccines, we have demonstrated that our VLPs, expressed in the cells of the person being vaccinated, are extremely safe, while eliciting both strong and durable humoral and cellular immune response. (Humoral immunity produces an antibody-mediated immune response whereas cellular immunity produces a cell-mediated immune response. B cell mainly regulates the humoral immunity whereas T cell regulates the cellular immunity.)
VLPs mimic the form of viruses and thereby train the body's immune system to recognize the authentic virus should it appear. VLPs also train the immune system to recognize and kill infected cells to control infection and reduce the length and severity of disease. When VLPs for viruses like COVID-19, Ebola, Marburg, Lassa fever or HIV are produced in vivo, they include not only the protein antigens, but also consist of membranes from the vaccinated individual's cells displaying vaccine proteins. In this way, they are highly similar to the virus generated in a person's body during a natural infection. VLPs produced externally, by contrast, are less familiar to the immune system due to the manner in which they are produced. Finally, and of critical importance, by producing VLPs in vivo, we avoid cumbersome purification issues associated with in vitro production of VLPs.
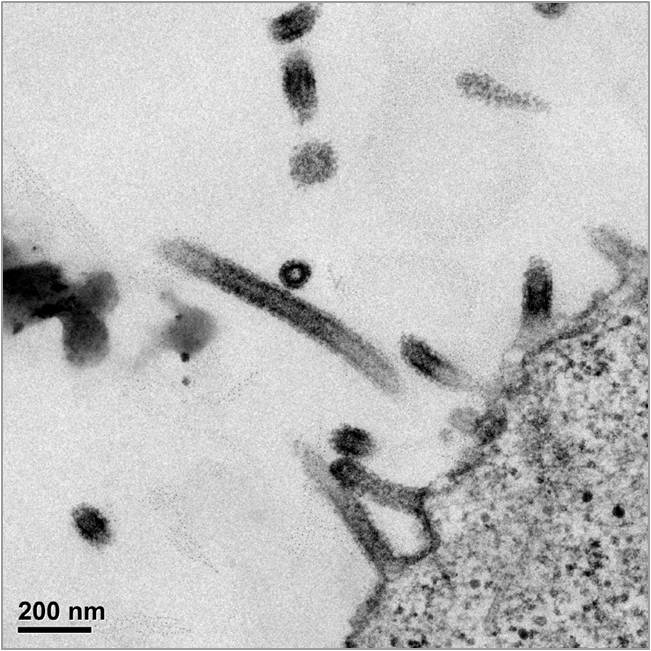
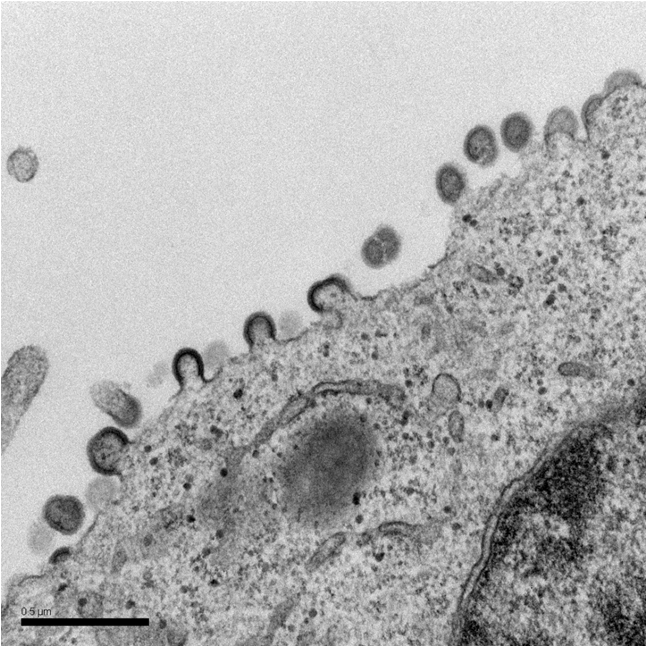
Ebola VLPs HIV VLPs
Examples of electron micrographs showing the VLPs elicited by Healthcare & Life Sciences Limited vaccines from human cells. Note that the Ebola VLPs on the left self-assemble into the rod-like shape of the authentic Ebola virus, while the HIV VLPs shown on the right take on the spherical shape of the authentic HIV virus. While below the resolution of these micrographs, both types of VLPs display the native form of their respective viral envelope glycoproteins which we believe is key to generating an effective immune humoral response.
Our MVA-VLP vector affords other unique advantages:
Safety: Safety for MVA has been demonstrated in more than 120,000 subjects, including immunocompromised individuals, during the initial development of MVA and more recently with the development of MVA as a safer vaccine against smallpox.
Durability: Healthcare & Life Sciences Limited/NIAID rMVA technology raises highly durable vaccine responses. We believe that elicitation of durable vaccine responses is conferred on responding B cells by the vaccinia parent of MVA, which raises highly durable responses for smallpox.
Limited pre-existing immunity to vector: Following the eradication of smallpox in 1980, smallpox vaccinations subsequently ended, leaving all but those born before 1980 and selected populations (such as vaccinated laboratory workers, first responders) unvaccinated and without pre-existing immunity.
Ability to use vector for multiple targets: Our vaccines against difference indications can be given simultaneously or as little as a few weeks apart.
No need for adjuvants: MVA stimulates strong innate immune responses and does not require the use of adjuvants.
Thermal stability: MVA is stable in both liquid and lyophilized formats (> 6 years of storage).
Genetic stability and manufacturability: If appropriately engineered, MVA is genetically stable and can reliably be manufactured in either the established Chick Embryo Fibroblast cell substrate, or novel continuous chicken or duck cell lines that support scalability as well as greater process consistency and efficiency.